Ultrasound guided steroid injections
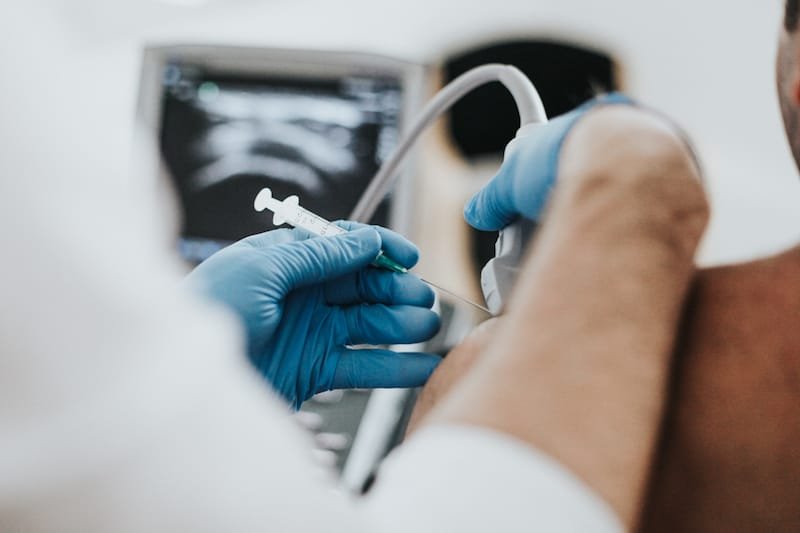
Pain relieving injections, such as corticosteroid and hyaluronic acid, are a very safe and effective treatment for a range of musculoskeletal conditions. In recent years more of these injections are being performed under ultrasound guidance to further improve safety and treatment outcomes. This article looks at the rationale and the research evidence behind this change.
Ultrasound uses high frequency sounds to create images of the tissues within the body. During injection procedures the needle can also be clearly visualised after it has entered the tissues of the body and therefore allows for accurate needle placement and delivery of the injection.
The use of glucocorticosteroid (also known as ‘corticosteroid’ or ‘steroid’) injections to reduce pain was first pioneered in the early 1950’s. There are now thought to be over half a million steroid injections performed in the UK in primary care each year (Maricar 2013).
Corticosteroid injections (CSI) are used to treat a range of musculoskeletal pain conditions ranging from osteoarthritis, inflammatory diseases and a wide range of commonly occurring conditions such as tendinopathic disorders and nerve impingements. In recent years an increasing number of these injections are now being performed using ultrasound guidance. The hypothesis being that there are advantages in having verification of needle placement and more precise delivery of the injection. This will improve treatment outcomes and reduce some of the risks associated with the procedure.
There have been a number of factors responsible for this shift towards ultrasound guided CSI (Davidson 2011). First, advancements and developments in diagnostic ultrasound machine technology have meant that image quality and definition has improved vastly in the last 15-20 years. At the same time technological advancements and competition in the market place has made diagnostic ultrasound machines more affordable and more user friendly. The use of ultrasound imaging has greatly expanded in a number of medical fields. In orthopaedic and musculoskeletal medicine, the use of dynamic ultrasound as a diagnostic tool and as an aid to injection interventions has been rapidly growing as a result.
There are a number of reasons, often highlighted in the literature, why performing steroid injections with the aid of ultrasound guidance might rationally be thought desirable and advantageous. Prior to this, the traditional ‘anatomical’ or ‘surface marked’ method whereby needle placement technique was determined by pre-set protocols based upon normal anatomical relationships have been utilised by orthopaedic doctors, rheumatologists and general practitioners for over half a century and accounted for a significant proportion of treatments for musculoskeletal pain management. Using ultrasound guidance is thought to significantly improve accuracy (although whether this leads to an improvement in treatment outcome however is not always clear). Ultrasound guidance is also thought to reduce the risk of injury and other potential side effects (Bloom et al 2012). Ultrasound may help identify abnormal anatomy / normal variants and also allow further clinical assessment of the target structure and surrounding tissue which may impact upon the clinical reasoning regarding the procedure and overall treatment and management.
With growing levels of litigation in many areas of medicine, obtaining images demonstrating accurate needle placement could have clear benefits. The improved accuracy may allow for more confident diagnosis based upon response to injection and may also reduce unnecessary injections after a failed treatment as a result. Increasingly, ultrasound guidance may have a more crucial role in the expanding repertoire of injection procedures that may be more reliant on accurate needle placement – hydrodistension procedures, barbotage and high-volume tendon stripping procedures are more sophisticated techniques requiring very specific needle placement and manipulation of the target tissues. Indeed, joint fluid aspiration often performed prior to steroid injection has been shown to be vastly more effectively and efficiently performed with guidance (Sibbitt, 2009). Finally, ultrasound guidance can provide a safe and easily accessible alternative to other forms of needle guidance such as fluoroscopy traditionally employed for deeper structures such as the hip joint.
The aim of this article is to consider a more in-depth appraisal of the evidence for these points.
Conclusions The findings of this position statement indicate there is strong evidence that USGIs are more accurate than LMGI, moderate evidence that they are more efficacious and preliminary evidence that they are more cost-effective. Furthermore, ultrasound-guided (USG) is required to perform many new, advanced procedures and will likely enable the development of innovative USG surgical techniques in the future.
Does Ultrasound Guidance Improve Accuracy?
There are numerous studies which have repeatedly demonstrated and confirmed that needle placement into target structures have a greater degree of accuracy and are successfully placed within the target structure on a much higher percentage of attempts when performed with ultrasound guidance (Berkoff 2012, Cunnington 2010, Patel 2012, Peck 2010, Smith 2010 & 2011). In a systematic review by Maricar et al (2013), ‘Where and how to inject the knee’, the potential benefits of ultrasound guidance were considered specifically for knee injections. Of the eight identified approaches for injection, improvements were greatest where clinicians injected using the mid-medial patellar or the anterior-lateral joint line approaches. There was a correlation between accuracy rates and level of clinical experience. Overall inaccuracy rate was 20% for blind injections. Curtiss et al 2011 reported accuracy ranging from 55-100% using surface marked techniques compared with range of 90-100% accuracy with image guidance. As ultrasound guided injections have fairly uniform high levels of accuracy the greatest differential is seen with joints and target structures that have low levels of accuracy with surface marked procedures. For example, Finnoff et al in their 2010 study found injections of the pes anserine to have 17% accuracy with surface marking versus 92% accuracy under ultrasound guidance.
Does Ultrasound Guidance Improve treatment outcomes?
The precise efficacy of steroid injections in general is still unclear from the research. There is some consensus that the efficacy appears better in acute, rather than chronic, conditions. Studies have demonstrated pain relief ranging from several days up to nine months (Arroll & Goodyear 2005). There is an ongoing debate with regard to the impact and clinical relevance of local administration.
There is a general consensus in the literature that corticosteroid injections give relief for up to 10 weeks following injection. There are very few studies that measure more long-term treatment outcomes.
Having established that ultrasound guidance improves accuracy, the evidence for improving treatment outcomes is less convincing. A number of studies show mild to moderate benefits (Berkoff 2012 – knee injections, Sibbitt et al 2012 – knee injections, Ucuncu 2009 – subacromial injection, Lee 2009 – shoulder injection). Short term treatment outcomes were shown to be favourable in studies looking specifically at knee joint injections but with no change in longer term outcomes. Sibbitt et al (2011) also reported better results from joint aspiration using ultrasound guidance. In Sibbitt’s (2009) article, the volume of aspiration fluid was 300% greater, patients reported 58.5% less pain at 2 weeks and 43% less procedural pain. The study also considered a range of joints with around 42% in the study being knees.
Marginal benefits should also be considered in relation to the overall additional costs incurred in setting up and providing services for ultrasound guided treatments (Sibbitt et al 2011). With only small margins of improvement compared with landmark guided injections, many researchers continue to question the cost effectiveness of any improvements in outcomes (Bolton et al 2018, Mitchell 2018, Bhayana 2018) and suggest first line treatment for many conditions does not require ultrasound guidance as the added cost is not justified.
The mechanism of action for corticosteroids
Currently, the exact mechanism of corticosteroid action as a locally acting analgesic is not fully understood. Steroids can be administered locally and systemically. They are believed to work by altering DNA gene transcription and production of proteins and enzymes. The subsequent anti-inflammatory and immunosuppressive effect typically can be detected within 4-6 hours post injection and continues after the drug has been cleared.
The original hypothesis for performing a local injection of steroid was to deliver a high concentration of the drug immediately adjacent to the suspected origin of pain (or pain producing structure) to assist in reduction of inflamed and oedematous tissue. It was also anticipated that there would be a local blockade of activated cytokines and immunological mediators. Therefore, because corticosteroid is able to dissipate through local tissue the measurable clinical gain through increased accuracy of delivery might only be marginal.
Any improvement in treatment outcome might also be evaluated in terms of the overall cost effectiveness of marginal benefits. However, with the expansion of injection treatments for musculoskeletal conditions including hyaluronic acid, local anaesthetic (as a treatment in its own right) and high volume procedures more research is required as to the clinical benefits of performing these injections with ultrasound guidance as their treatment effects might be more dependent upon accuracy than with steroid injections alone.
Does ultrasound guidance reduce the risk of side effects from steroid injections?
Performing steroid injections allows the total treatment dose to be less than if treating by systemically acting oral steroids (Rogojan 2004, Nichols 2005). Whilst theoretically there is still potential for systemic adverse effects (endocrinal, metabolic, hematologic and vascular) (Kumar 1999, Lavelle 2007, Habib 2009, Brinks 2010) the risks are significantly reduced.
Steroid injections can also cause local adverse effects such as local pain, haemorrhage, ulceration, atrophy, pigmentary changes, calcification, secondary infection, granuloma formation, Charcot’s atrophy, avascular necrosis, Nicolau’s syndrome or tendon rupture (Kumar 1999, Canturk 2004, Papadopoulos 2009, Habib 2010, Brinks 2010). A recent Cochrane review (Bloom 2012) found 9% of patients injected under ultrasound guidance had suffered minor adverse effects, such as post-injection pain, compared with 15% of patients receiving blind or ‘surface marked’ injections.
Serious adverse side effects from steroid injections are relatively rare. Joint infections following injections are thought to occur in approximately 1/10,000-1/50,000 procedures (Rosen 2017, Rogojan 2004). However localised changes to skin pigmentation and subcutaneous fat atrophy caused by steroid are thought to occur in around 1-8% of localised steroid injections (Kumar 1999, Canturk 2004, Papadopoulos 2009, Habib 2010, Brinks 2010). These local side effects can be particularly problematic when injecting superficial structures such as De Quervain’s tenosynovitis of the wrist (Jeyapalan 2009), trigger finger (Akhtar 2005) ganglion (Breidahl 1996), tennis elbow (lateral epicondylitis) (Kumar 1999) and structures around the foot such as plantar fasciitis (Tsai 2005, Yucel 2009, Tatli 2009, McNally 2010). Although these effects do not generally cause any serious medical complications, fat atrophy around the heel pad of the foot can lead to irreversible complications such as persistent pain (Tatli 2009).
Changes in local tissues from steroid are believed to be often related to sub-optimal needle placement which may lead to steroid being deposited within the subcutaneous fat. Most significant to the use of ultrasound guidance is the positioning and accuracy of the injection procedure. There exists a strong body of evidence that ultrasound guidance can significantly improve the accuracy of intra-articular injections (Rutten et al 2007, Chen 2006) and the ability to aspirate more effectively from smaller joints (Balint et al,2002) suggests the control and accuracy of needle tip placement is significantly improved by ultrasound guidance. Ultrasound can therefore allow the practitioner to judge and control the depth of the needle before depositing the steroid which would potentially reduce risks of depositing steroid in the wrong place. Sawaizumi et al 2007 showed 36% of patients suffered local complications including depigmentation after injection for De Quervains tenosynovitis compared with none when performed with ultrasound guidance.
Ultrasound as a means of improving accuracy of needle placement has significant advantages over other techniques for guiding needle placement such as fluoroscopy guidance. Ultrasound is relatively cheap, easily accessible and well tolerated by patients. Ultrasound has virtually no contra-indications to usage and is not known to have any harmful side effects.
There are some potential pitfalls when using ultrasound guidance that can actually raise the risks of adverse effects. For example, some practitioners in the past were taught a technique of releasing a small bolus of the steroid to help allocate the needle tip. If this were performed too superficially or within the heel pad of the foot, it could lead to adverse effects. Some ultrasound guided techniques require the needle to travel a longer distance to the target tissue. This requires careful consideration when considering depth of structure and selecting equipment for the procedure.
Sites et al (2004) considered the learning curve when developing the skills to perform guided procedures and suggested that, until significant experience had been gained, performing ultrasound guided injections may pose greater risks to the patient than performing injections using the surface marking technique.
Brinks et al (2010) in their systematic review article on the adverse effects of extra-articular corticosteroid injections conclude that adverse events with corticosteroid injection can be minimised by ensuring appropriate procedures are followed by well trained and competent practitioners.
Ultrasound guidance may also have a significant role in reducing risks to patients in the hands of dedicated and highly trained, experienced clinicians with a commitment to clinical excellence and delivering the highest quality treatment to their patients.
Could ultrasound assessment help diagnosis and patient selection for steroid injection?
There have been several pieces of research attempting to identify clinical features associated with positive response to steroid injection treatment.
Maricar et al (2013), in his review article critically appraised the evidence for possible predictive factors relating to outcome from steroid injection. Signs of effusion were generally regarded as being associated with a good response to steroid injections. The presence of synovitis was less clear and the evidence was mixed. The severity of osteoarthritic changes such as those shown on X-ray were shown to have poor correlation with pain and poor levels of prediction of outcome from steroid injection. A study by Stitik (2011) demonstrated underlying calcium, pyrophosphate deposit disease (eg. pseudo gout or chondrocalcinosis) generally respond well to steroid injection. These changes can potentially be identified on ultrasound (Dufauret-Lombard 2010, Thiele 2007). Sibbitt et al (2011), in their article in the Journal of Rheumatology, comment that “a randomised control trial of the cost effectiveness of ultrasound guided intra-articular injection of inflammatory arthritis showed a significantly improved outcome in the reduction in injection pain and increase in the therapeutic duration in the 124 knees injected under ultrasound guidance, compared with the 120 injected with conventional palpation guided anatomic injection and conclude that sonographic needle guidance improves the performance, clinical outcomes and cost effectiveness of intra-articular injection for inflammatory arthritis.”
Clinicians might also argue that performing diagnostic ultrasound at the time of the injection might identify factors that might make a steroid injection seem less desirable or less appropriate as a possible treatment. The presence of severe degenerative changes of a tendon might contraindicate injection or change the clinician’s approach to treatment. Therefore, the ultrasound findings may be key in clinical decision making associated with patient safety and reducing the risk of adverse effects of injection, such as tendon rupture.
Conclusions
| Method | Accuracy | Outcome | Safety | Tolerance | Accessibility |
|
Ultrasound Guided |
Highly accurate |
Better outcomes |
Reduce risks |
Well tolerated |
Fast access |
|
Surface Marked |
Poor accuracy |
Good outcomes |
Nil reduced risks |
Well tolerated |
No access issues |
|
CT/X-ray guided |
Highly accurate |
Good outcomes |
Reduce some risks |
Not always well tolerated |
Limited access / increased costs |
To evaluate the benefits of using ultrasound for performing corticosteroid injections there are several areas to consider. Research has shown a significant improvement in accuracy for ultrasound guided steroid injections. Nearly all ultrasound guided injections show high levels of accuracy, generally >90-95%. The greatest benefit in relative accuracy is found when comparing with ‘blind’ or ‘surface marked’ procedures with low levels of accuracy such as small joints of the hand and small target structures such as pes anserine injections (Finnoff 2010). There is some evidence that ultrasound guided injections give better outcomes and the benefits last for longer. Due to the specific mechanisms of action of corticosteroid in reducing pain the precise location of the drug in relation to target structure may be less critical. There have been no studies to date to investigate whether treatment dose could be reduced for ultrasound guided procedures. It is also important to note that many procedures such as barbotage, hydrodistension injections, hyaluronic acid and platelet rich plasma (PRP) injections are likely to be far more reliant upon precise targeting of the needle tip. These procedures often include a specific mechanical aspect to the treatment such as stretching and distending structures which requires highly accurate needle tip placement and the ability to visualise and monitor the impact on structures as the treatment is delivered.
There is evidence that ultrasound guidance reduces local side effects and tissue injury relating to corticosteroid injections and this might be as a result of accurate needle placement and avoidance of particular structures. Also, ultrasound evaluation may aid with diagnosis and clinical reasoning in relation to appropriate treatment selection.
There is overwhelming evidence to support the various ways in which ultrasound guidance improves the safety and accuracy of steroid injection treatment. The potential to reduce the risks of local tissue injury including nerve and vascular injury alone warrants the use of ultrasound guidance wherever possible. The feedback we receive from patients also reassures us that patients really feel much safer and more assured when they have an ultrasound guided injection. We strongly believe that this should be the universal gold standard for giving patients the best possible treatment and care.
Article by Dave Baker – Clinical director Complete Injections
References
Arroll, B., Goodyear-Smith, F., (2005) Corticosteroid injections for painful shoulder: a meta-analysis. BJGP March 1, 2005 vol. 55 no. 512 224-228
Bellamy, N., et al (2005) Intra-articular corticosteroid injection for treatment of osteoarthritis and the knee (Review) Cochrane Database Systematic Review 2:CD005328
Caldwell, J., (1996) Intra-articular Corticosteroids – Guide to selection and indications for use. Drugs: Oct: 52 (4), 507-514.
D’Agostino, Maria-Antonietta, and Wolfgang A. Schmidt. “Ultrasound-guided injections in rheumatology: actual knowledge on efficacy and procedures.” Best Practice & Research Clinical Rheumatology 27.2 (2013): 283-294.
Daniels, Eldra W., et al. “Existing Evidence on Ultrasound-Guided Injections in Sports Medicine.” Orthopaedic journal of sports medicine 6.2 (2018): 2325967118756576.
Dufauret-Lombard, C., Vergne-Salle, P., Simon, A., Bonnet, C., Treves, R. and Bertin, P., 2010. Ultrasonography in chondrocalcinosis. Joint Bone Spine, 77(3), pp.218-221.
Finnoff, Jonathan T., et al. “American Medical Society for Sports Medicine (AMSSM) position statement: interventional musculoskeletal ultrasound in sports medicine.” PM&R 7.2 (2015): 151-168.
Finnoff, J.T., Nutz, D.J., Henning, P.T., Hollman, J.H. and Smith, J., 2010. Accuracy of ultrasound-guided versus unguided pes anserinus bursa injections. PM&R, 2(8), pp.732-739.
Gaffney, K., et al (1995) Intra-articular triamcinolone hexacetonide in knee osteoarthritis: factors influencing the clinical response. Ann Rheum Dis. 54:379-81.
Job-Deslandre, C., et al (1990) Administration of methylprednisolone pulse in chronic arthritis in children. Clinical and Experimental Rheumatology 1991, 9 Suppl 6:15-18
Maricar, N., et al (2013) Predictors of response to intra-articular steroid injections in knee osteoarthritis – a systematic review. Rheumatology: 52: 1022-1032.
Ostergaard, M., Halberg, Poul., (1998) Intra-articular corticosteroids in arthritic disease: A guide to treatment: Feb: 9 (2): 95-103.
Pendleton et al (2008) Can sonography be used to predict the response to intra-articular corticosteroid injection in primary osteoarthritis of the knee? Scand J Rheumatol: 37: 395-397
Pyne, D., et al (2004) Intra-articular steroid in knee osteoarthritis; a comparative study of triamcinolone hexacetonide and methylprednisolone acetate. Clinical Rheumatology. 23 (2);116-20
Rosen, R.C., Stuto, A.C. and Cook, K.D., Group a Streptococcus Necrotizing Soft Tissue Infection Secondary to Corticosteroid Injection: A Case Report and Literature Review. Clin Surg. 2017; 2, 1431.
Sinatra, R.S., Jahr, J.S., Watkins-Pitchford (2011) The Essence of Analgesia and Analgesics.pp 384-387 Cambridge Medicine
Stitik, T.P., (2011) Injection Procedures ; Osteoarthritis and Related Conditions. Springer Science and Business Media, New York
Thiele, R.G. and Schlesinger, N., 2007. Diagnosis of gout by ultrasound.

