Generally speaking, corticosteroid injections are very safe, but there are some rare occasions when a corticosteroid injection is not appropriate or require additional considerations. It is therefore important that every patient is assessed individually.
This blog discusses medical conditions which can affect corticosteroid injection therapy.
If you are unsure as to whether an injection is right and appropriate for you please contact us on 0207 482 3875 or email injections@complete-physio.co.uk.
Absolute contraindications to injection therapy
1. Corticosteroids and allergic reactions
It is very rare to have an allergic reaction to corticosteroid or to local anaesthetic (Lidocaine and Marcaine are the most commonly used local anaesthetics in the UK). However, if you know you have an allergy to corticosteroid then an injection cannot be offered. If you have a known allergy to local anaesthetic, then a corticosteroid injection can still be undertaken without. This might make the actual procedure slightly more uncomfortable but will not affect the efficacy of the corticosteroid.
2. Corticosteroids and infection
Infection is an incredibly rare side effect associated with corticosteroid injections with rates of septic arthritis following injection reported to be as low as 1/50,000 (Chung et al., 2020). There are strict evidence-based policies and procedures set by the National Institute for Health and Care Excellence (NICE, 2012) which are designed to limit post injection infections. These state that the area to be injected should be cleaned and sterilised thoroughly and that an ‘aseptic’ ‘no touch’ injection technique must be used (Draghi et al., 2010; Holland et al., 2012). In the case of ultrasound guided injections the application of sterile gel (used to maintain an ultrasound image during the procedure) must be used (UK Health Security Agency, 2021). At Complete Injections we follow the most up-to-date guidelines and policies to limit the risk of infection. All our clinicians are infection control trained, use sterile ultrasound gel, and adopt the Aseptic No-Touch Technique (ANTT) with every procedure.
Patients at the highest risk of septic arthritis (most commonly caused by the staphylococcus aureus pathogen) after a corticosteroid injection are those with pre-existing medical conditions such as:
- Diabetes
- Chronic renal disease
- A prosthetic joint
- Rheumatoid and osteoarthritis
- Immunosuppressed patients (for example HIV)
- Intravenous drug users (Chung et al., 2020)
Whilst the guidelines surrounding infection control are extremely effective at limiting infection rates, there is still a very small chance that an infection may occur. If an infection is suspected, then it is vital that you attend A&E for assessment.
Signs of infection are:
- A significant increase in pain. The most prevalent symptom associated with infection (Chung et al., 2020).
- Local redness, heat and swelling
- Suddenly feeling unwell
- A fever
To further limit risk of infection there are some situations when a corticosteroid injection is contraindicated. These are outlined below.
3. Signs of skin infection or loss of skin integrity at the site of the proposed injection
Undertaking an injection when there is a loss of skin integrity (a break in the skin) or signs of a local infection (redness, swelling, heat) increases the risk of post injection infection and is therefore contraindicated (Cardone et al., 2002; Draghi et al., 2010; Kompel et al., 2019). Once the skin has completely healed and any signs of local infection have cleared then a corticosteroid injection can be administered.
4. Corticosteroids and antibiotics
As previously mentioned, corticosteroids have immunosuppressive properties and therefore, if you are currently taking antibiotic medication for an active infection, a corticosteroid injection cannot be administered (MacMahon et al., 2009). It is important that the full course of the antibiotic medication is completed and that you are clear of any residual signs and symptoms of infection prior to receiving a corticosteroid injection.
5. Corticosteroid injections and Surgery
Injecting corticosteroid either prior to or just after surgery (in particular joint replacement) has been linked with increased rates of joint infection (Xing et al., 2014; Simurina et al., 2019; Roecker et al., 2019).
Corticosteroid is an immunosuppressant medication and is thought to increase the risk of infection in subsequent arthroplasty by not fully dissolving within the joint space. Any residual corticosteroid in a joint has the potential to maintain an immunosuppressant response, leaving the joint susceptible to infection.
Infection after surgery is a rare occurrence but when it does occur it can be catastrophic, resulting in loss of functional capacity and increased rates of mortality.
Corticosteroid injections can be effective at controlling the pain associated with hip and knee osteoarthritis, providing a ‘window of opportunity’ for the patient to engage in a physiotherapy rehabilitation program. It is not possible to predict which patients will benefit from an injection or who might suffer an infection, but administering a corticosteroid injection to any patient who may in the near future require surgery is an absolute contraindication.
The cause of increased infection rates seen in this patient cohort is not fully understood. Infection can be caused by a plethora of mechanisms including the steroid medication itself, the local anaesthetic (used in the injection procedure), the mechanism of the needle passing into the joint space, or a breech in sterility of the injection procedure (Xing et al., 2014). It is therefore of the utmost importance that all injections are undertaken using an aseptic no-touch procedure to minimise risk.
If you are on a waiting list for a joint replacement, a corticosteroid injection is not advised. Consensus amongst orthopaedic consultants suggests no corticosteroid injections should be carried out in the preceding nine months prior to joint replacement surgery. If you are experiencing increasing pain during this period, it is highly recommended that you discuss your situation with your orthopaedic consultant who can help you make a decision on the best management plan for you. Any decision to undertake a pre-surgical corticosteroid injection must be discussed with and performed by your orthopaedic team.
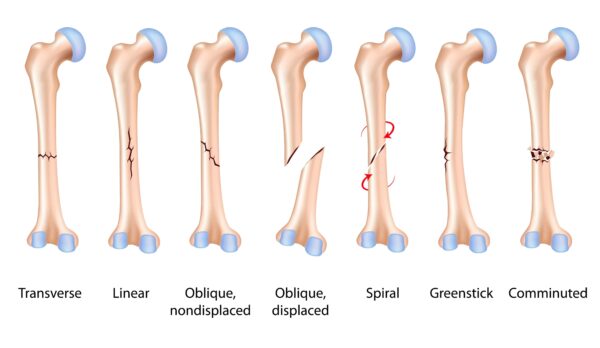
6. Corticosteroids and Trauma (fractures and hemarthrosis)
Injecting a traumatic injury is an absolute contraindication. Corticosteroid medication has been shown to inhibit bone healing and therefore administering a corticosteroid injection in the presence of a fracture must be avoided (Cardone et al., 2002; MacMahon et al., 2009; Kompel et al., 2019). If your pain and symptoms started after a traumatic event, then an Xray is required to rule out the possibility of fracture. Furthermore, the presence of a hemarthrosis (blood within a joint) positively correlates with an intraarticular fracture and therefore an injection cannot be undertaken. Diagnostic ultrasound is capable to assessing the joint for possible hemarthrosis. All our clinical specialists are experienced in assessing joints using diagnostic ultrasound. If a hemarthrosis is suspected and onward referral to A&E for further investigations is required.
7. Corticosteroids and bone density
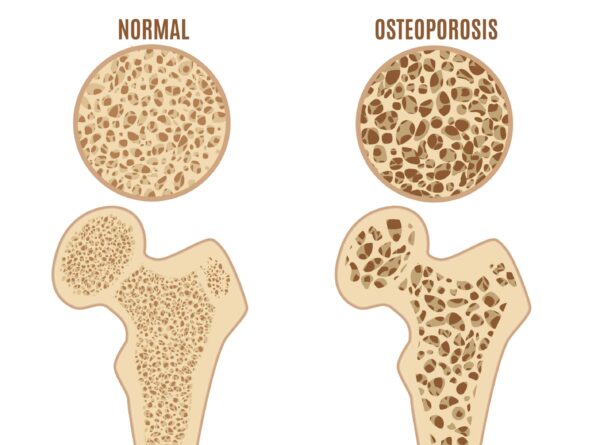
If there is evidence of juxta-articular osteoporosis (a focal area of osteoporosis located adjacent to or within the joint) then a corticosteroid injection is not advised. This is due to the increased risk of reducing bone density further, and increasing joint damage (MacMahon et al.,2009; Simurina et al., 2019).
8. Corticosteroids and joint instability
An already unstable joint can be made worse if infiltrated with corticosteroid. This is thought to be due to the development of subchondral osteonecrosis (breakdown of the sub chondral bone surface due to reduced blood flow) and a progressive weakening of the joint capsule and associated ligaments (MacMahon et al.,2009; Simurina et al., 2019).
9. Adverse drug reactions related to corticosteroids

An adverse drug reaction can occur if two opposing drugs are taken at the same time. For example convulsions have been reported in patients taking ciclosporin after receiving an injection of methylprednisolone (commonly known as Depomedrone), a corticosteroid commonly used to treat musculoskeletal conditions. Due to this adverse drug reaction patients taking ciclosporin cannot be offered an injection using Depomedrone.
10. Corticosteroids and under 18s
At complete we do not injection children. Generally speaking musculoskeletal pathology in the under 18s responds very well to rest and physiotherapy. Children’s skeletal systems are constantly developing and growing and therefore heal very rapidly. Corticosteroid injections, especially into joints, is contraindicated due to the risk of affecting joint and soft tissue development. Furthermore, all the research we have surrounding corticosteroid injections has been undertaken on adults. We therefore, we are not able to comment on the safety profile of corticosteroids in the paediatric population.

Systemic drug absorption can occur following a corticosteroid injection and therefore both local and global effects should be expected. There are some situations when a corticosteroid injection is indicated but the presence of concurrent medical conditions requires careful additional consideration.
If you are taking anticoagulant medication, are a diabetic, have glaucoma, are pregnant, are being actively treated for cancer or have had multiple recent corticosteroid injections then additional considerations are necessary if an injection is to be completed safely and effectively. If deemed as safe to undertake a corticosteroid injection, then a period of additional post injection monitoring is often required.
For further information please see below:
1. Anticoagulant medication (blood thinners)

A corticosteroid injection can be offered to patients taking anticoagulation medication, but careful consideration is required due to the increased risk of local bleeding (Cardone et al., 2002; Draghi et al., 2010; Simurina et al., 2019).
A large systematic review was conducted to assess the risk of bleeding after a corticosteroid joint injection. Results revealed that joint injections are safe to undertake whilst continuing to take rivaroxaban, apixaban and dabigatran (Tarar et al., 2021). Warfarin or heparin, two commonly used anticoagulant medications, were not included in this review but common guidelines suggest that an INR (blood clotting ratio) of under 3.0 is safe.
If you are on high doses of rivaroxaban, apixaban and dabigatran then your GP might request that you stop taking your medication for a short period (usually 24 hours) prior to the injection (Draghi et al., 2010). If your coagulation status is unstable or your INR higher than 3 then a corticosteroid injection may be contraindicated. However, this should be discussed directly with your GP (Bashir et al., 2015; Kompel et al., 2019).
Some patients do require an INR greater than 3, namely patients with specific types of prosthetic heart valves. In this situation, if a corticosteroid injection is the treatment of choice, then specialist referral to an orthopaedic team is required. If you are concerned about your anticoagulant status, it is advisable to discuss this with your GP if a corticosteroid injection is being considered.
2. Uncontrolled Diabetes

Careful consideration must be applied when contemplating a corticosteroid injection for diabetic patients due to the associated risk of transient hyperglycaemia (elevated blood sugar levels) (Kompel et al., 2019). Hyperglycaemia was reported to be present in the first two days post injection with blood sugar levels returning to normal by day three. However elevated blood sugar levels can persist for a few weeks. Diabetic patients most affected by transient hyperglycaemia were type one diabetic patients and patients taking insulin (Jeffery et al., 2014). It is therefore important that diabetes is well controlled prior to receiving an injection, and monitored closely after the procedure. At Complete we ask that patients with an HbA1C of >8.5% get consent from their G.P prior to having a corticosteroid injection. For more information of Diabetes and musculoskeletal pain please follow this link.
3. Corticosteroids and Glaucoma
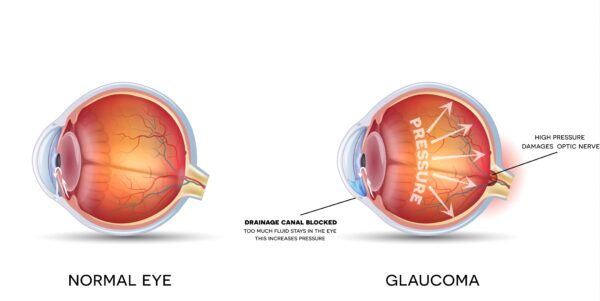
Research has revealed the risk of an increase in intraocular pressure following a corticosteroid injection. There remains speculation surrounding how long the increased pressure lasts or the long-term implications of this raised pressure (Taliaferro et al., 2018; Kersey, 2004). If you have a diagnosis of glaucoma, then it is important that your intraocular pressure is recorded prior to injection and that your optician has deemed it safe for you to receive an injection.
4. Corticosteroids and pregnancy.

Medication given to the mother has the potential to pass to the unborn child and therefore , corticosteroid injections whilst pregnant cannot be undertaken. Currently there is not enough research available to prove or disprove the safety surrounding a corticosteroid injections and pregnancy.
5. Corticosteroids and breastfeeding
You are able to have a corticosteroid injection whilst breastfeeding. However, careful consideration is required before undertaking an injection. Research discussed by the breast feeding network into corticosteroid injections whilst breastfeeding suggests that corticosteroid injections can be safely administered whist breastfeeding. With this in mind undertaking a corticosteroid injection whilst breastfeeding still does require consideration. Taking into account the level of pain, the risks and benefits of undertaking the procedure, the medication dose levels, and the medical status of the mother and the child are all crucial considerations (Bauchat et al., 2018).
6. Repeated corticosteroid injections
Research has suggested that multiple corticosteroid injections targeting the same joint can be harmful however there is minimal research suggesting that multiple corticosteroid injection actually cause harm to a joint. With this in mind, common consensus suggests that three injections for the same condition in a year is a safe amount, with further injection therapy requiring careful consideration (MacMahon et al., 2009; Saunders., 2012; Simurina et al., 2019). The aim of a corticosteroid injection is to reduce pain, allowing you a window of opportunity to address the underlaying condition. It is essential that a course of physiotherapy be completed after a corticosteroid injection. It may be possible to reduce the need for subsequent injection if you are able to maintain a strong and flexible joint. For more information please follow this link.
7. Corticosteroids and HIV medication

Ritonavir is a common medication used to treat HIV patients however, there is potential for adrenal suppression when combined with a corticosteroid. If you are being treated with Ritonavir then it is important that you have discussed having a corticosteroid injection with your consultant and that they have deemed it safe and appropriate for you to have an injection.
8. Corticosteroid injections and cancer

If you have a history of cancer or are currently being treated for active cancer then a corticosteroid injection my not be appropriate. Prior to undertaking a corticosteroid injection your G.P and oncologist will need to be consulted to ensure it is a safe option for you. An X ray or MRI may be required to ensure the pain you are experiencing is musculoskeletal in nature and not due to your cancer diagnosis, especially if your symptoms came on very quickly and are significant.
If you are currently being treated for cancer your oncologist will need to give consent for a corticosteroid injection. The effectiveness of many medications used to treat cancer can be diminished if subjected to corticosteroid.
Corticosteroid injections remain a safe and effective method of controlling musculoskeletal pain, allowing patients to undertake activities of daily living and leisure activities more comfortably. They can provide a ‘window of opportunity’, allowing a period of pain free rehabilitation to take place to address the underlying condition. However, careful consideration is required to ensure a safe and effective injection is undertaken.
At Complete Injections we do everything we can to ensure a safe and effective experience for patients. Our clinicians are fully trained in life support (CPR) and anaphylaxis management. Our clinics are also equipped with defibrillators and stocked with adrenaline. Before you attend an appointment at Complete Injections you will be sent an extensive medical screening and consent form. One of our expert clinicians will assess your file prior to your appointment and if required you will receive a call to discuss any issues flagged up in your pre-injection paperwork. On the day of your appointment, you will be asked a series of medical questions to ensure that an injection is still a safe and appropriate treatment. A full physical assessment will be completed prior to a preliminary ultrasound scan. Once a formal diagnosis has been made your clinician will discuss with you the most effective treatment options. You will be informed of the pros and cons of the injection and what you might expect before, during and after the procedure. All injections undertaken at Complete Injections are ultrasound guided. This ensures that you receive the safest, most accurate, and effective treatment possible.
For further information or to book an appointment, please contact us on 0207 482 3875 or email injections@complete-physio.co.uk.
References
Bashir, M.A., Ray, R., Sarda, P., Li, S. & Corbett, S. 2015, “Determination of a safe INR for joint injections in patients taking warfarin”, Annals of the Royal College of Surgeons of England, vol. 97, no. 8, pp. 589-591.
Saunders, S., FCSP & Longworth, S. 2012, Injection techniques in musculoskeletal medicine: a practical manual for clinicians in primary and secondary care, 4th edn, Churchill Livingstone Elsevier, Edinburgh.
UK health security agency. Good infection prevention practice: using ultrasound gel, 2021